Abstract
Introduction: Total body irradiation (TBI) continues to be an important part of the conditioning regimen for allogeneic hematopoietic cell transplantation (allo-HCT) in acute lymphoblastic leukemia (ALL). Previous dose escalation studies showed that higher than 12-Gray (Gy) was toxic and did not provide any apparent survival benefit - at least in patients (pts) transplanted in first complete remission (CR1) - thus establishing 12-Gy as the standard TBI dosage. Whether 8-Gy instead of 12-Gy TBI is sufficient in ALL CR1, as has been prospectively demonstrated for AML CR1 (Lancet Oncol 2012; 13: 1035-1044), has not yet been studied.
Methods: In this registry-based retrospective study of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (ALWP-EBMT), we compared outcomes of ALL-CR1 pts who underwent a matched-sibling donor (MSD) or matched-unrelated donor (MUD) allo-HCT (94% peripheral blood stem cells) with TBI-based conditioning at a total dose of 12-Gy vs 8-Gy. Patients included in this analysis had received fludarabine (Flu) as the sole chemotherapy counterpart of TBI (12-Gy vs 8-Gy TBIFlu).
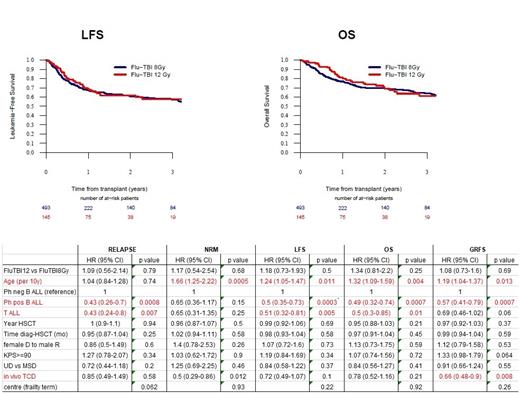
Results: The median follow up for the whole cohort (n=639 pts) was 22.5 months (95% CI, 17.2-24.1) and did not differ between the 8-Gy (n=494) and 12-Gy (n= 145) TBIFlu treated pts. 25% pts had B-precursor ALL, 54% Philadelphia (Ph)-positive ALL and 21% T-ALL (p=0.008 between groups). Patients conditioned with 8-Gy TBIFlu were older than 12-Gy TBIFlu treated pts (median 55.7 vs 40.3 years, IQR 50.2-61.3 vs 27-50.2 years, <0.0001) and more frequently received in vivo T-cell depletion (71% vs 40%, <0.0001). All other characteristics were well balanced between 8-Gy vs 12-Gy groups including time from diagnosis to HCT (5.5 vs 5.8 months), Karnofksy <90% (34% vs 26%), minimal residual disease (MRD) positivity at HCT (37% vs 43%), MUD (72% vs 68%) and type of GvHD prevention. Engraftment failure was low and below 2% in both groups. Overall, 29% and 27% of 8-Gy vs 12-Gy treated patients died, with the main causes of death not differing between groups (relapse 41% vs 44%, infections 26% vs 24%, GVHD 12.6% vs 12.7%, respectively). Both in univariate and in the age-adjusted Cox proportional-hazards analysis, relapse (REL), non-relapse mortality (NRM), leukemia-free survival (LFS), overall survival (OS), and GVHD-free, relapse-free survival (GRFS) were not influenced by TBI dose (Figure 1, Table 1). These results were confirmed when we focused on pts aged <55 years (median age 47 years; 8-Gy 229 pts vs 12-Gy 131 pts). In the multivariate analysis, an incremental age of 10 years was associated with increased NRM risk (hazard ratio [HR] 1.66, 95% CI, 1.25-2.22) and reduced OS (HR 1.32, 1.09-1.59). Ph+ and T-ALL pts had significantly better survival outcomes than Ph- B-ALL pts, mainly due to significantly fewer relapses (Table 1).
Conclusion: Although there were limitations to this study (TBI dose and age were correlated; missing data on TBI fractionation; missing MRD data for nearly one-third of the pts) this retrospective analysis was able to investigate the effect of TBI total dose independently from the chemotherapy counterpart (TBIFlu regimen only) and suggests that 12-Gy and 8-Gy results in similar outcomes in ALL patients transplanted in CR1. Whether this is also true for more advanced disease (>=CR2) and/or young adults) cannot be answered, as our study included only CR1 pts, few of whom were below 25 years of age. The reduced REL risk of Ph+ B-ALL pts is probably due to the increased use of tyrosine kinase inhibitors (TKIs) pre- and post-transplant. Clinically, these results suggest 8-Gy TBI as sufficient for ALL patients transplanted in CR1 with no additional benefit of augmenting the conditioning intensity to 12-Gy, a finding which should be validated in prospective trials.
Spyridonidis: Menarini: Current Employment. Labopin: Jazz Pharmaceuticals: Honoraria. Giebel: Janssen: Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Speakers Bureau. Peric: Therakos: Honoraria; servier: Honoraria; MSD: Honoraria; Astellas: Honoraria; NOVARTIS: Honoraria; Abbvie: Honoraria; Pfizer: Honoraria. Schönland: Janssen: Honoraria, Other: Travel grants, Research Funding; Pfizer: Honoraria; Prothena: Honoraria, Other: Travel grants; Takeda: Honoraria, Other: Travel grants; Sanofi: Research Funding. Kröger: Novartis: Research Funding; Riemser: Honoraria, Research Funding; Sanofi: Honoraria; Neovii: Honoraria, Research Funding; Jazz: Honoraria, Research Funding; Gilead/Kite: Honoraria; Celgene: Honoraria, Research Funding; AOP Pharma: Honoraria. Stelljes: Novartis: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Kite/Gilead: Consultancy, Speakers Bureau; MSD: Consultancy, Speakers Bureau; Medac: Speakers Bureau; Celgene/BMS: Consultancy, Speakers Bureau; Pfizer: Consultancy, Research Funding, Speakers Bureau. Schroeder: JAZZ: Honoraria, Research Funding. McDonald: BioCryst Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees. Ganser: Novartis: Honoraria; Jazz Pharmaceuticals: Honoraria; Celgene: Honoraria. Wulf: Novartis: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Clinigen: Consultancy, Honoraria. Bazarbachi: Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Hikma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal